Answer:
ΔT = 0.017 °C
Step-by-step explanation:
According to the given condition, the change in internal energy of the block must be equal to 85% of its kinetic energy:
Change in Internal Energy = (0.85)(Kinetic Energy)
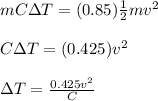
where,
ΔT = increase in temperature = ?
v = speed of block = 4 m/s
C = specific heat capacity of copper = 389 J/kg.°C
Therefore,
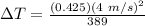
ΔT = 0.017 °C