Answer:
Zero-Order
Step-by-step explanation:
The exothermic decaying of nitrous oxide at 575° C will lead to
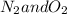 as follows:
as follows:
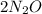 →
→
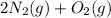
Hot platinum wire in the above reaction would function as a catalyst in the zero-order. However, if the reaction is considered in the gaseous phase, it will be more inclined towards second-order.
In the given scenario(
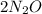 →
→
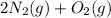 ), the reactant molecules of Nitrous oxide are restricted to the ones which have linked themselves to the catalyst's surface. Once this limited surface is filled, the extra molecules of gas would remain vacant until the previously attached molecules with the surface are decayed entirely.
), the reactant molecules of Nitrous oxide are restricted to the ones which have linked themselves to the catalyst's surface. Once this limited surface is filled, the extra molecules of gas would remain vacant until the previously attached molecules with the surface are decayed entirely.