Answer:
100. mL
Step-by-step explanation:
The computation of the volume in mL is shown below:
The balance equation of the double displacement reaction is
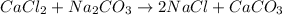
Now determine the moles that corresponding to 1.00 g of CaCO₃
As,
The molar mass of CaCO₃ is 100.09 g/mol.
So,
= 1.00 g × 1 mol/100.09 g
= 0.0100 mol
Now
the moles of CaCl₂ needed to generate 0.0100 moles of CaCO₃
As we know that
The molar ratio of CaCl₂ to CaCO₃ is 1:1.
So, The moles of CaCl₂ needed is
= 1 ÷ 1 × 0.0100 mol
= 0.0100 mol.
Now volume of 0.100 M CaCl₂ that contains 0.0100 mol is
= 0.0100 mol × 1 L/0.100 mol × 1000 mL/1 L
= 100. mL