Answer:
The answer is "Base".
Step-by-step explanation:
Given equation:
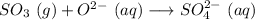
In the above-given chemical equation, the
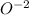 is the lewis base which is used to donate an electron pair to the central atom of
is the lewis base which is used to donate an electron pair to the central atom of
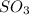 to form
to form
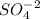 .
.
It is any substance like the
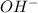 ion which might give a pair of non-bound electrons. Hence, it is an electron-pair donor. Throughout the theory of Lewis acid is an ion or molecule which accepts a pair of nonbonding electrons of value, that's why the base is the correct answer.
ion which might give a pair of non-bound electrons. Hence, it is an electron-pair donor. Throughout the theory of Lewis acid is an ion or molecule which accepts a pair of nonbonding electrons of value, that's why the base is the correct answer.