Answer:
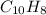
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to find the molecular formula of the given compound by firstly calculating both moles and grams of carbon in carbon dioxide and hydrogen in water, as the only sources of these elements derived from the compound x due to its combustion:
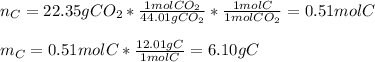
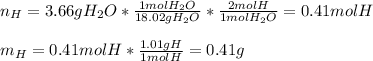
Now, since the addition of carbon and hydrogen is about 6.50 grams, we infer the compound has no oxygen, that is why we now set the mole ratios in the empirical formula for both C and H as shown below:
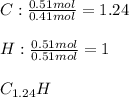
Yet it cannot be decimal, that is why we multiply by 4 to get the correct whole-numbered empirical formula:
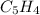
Whose molar mass is 64.09 g/mol, which makes the ratio of molar masses:
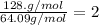
Therefore, the molecular formula is twice the empirical one:
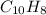
Regards!