Answer:
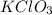
Step-by-step explanation:
Hello!
In this case, as we know the mass of the total sample, we can first compute the mass of oxygen:
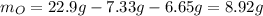
Next, we compute the moles of each element:
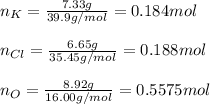
Now, we divide the moles by 0.184 moles, the fewest ones, to obtain:
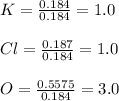
Therefore, the empirical formula is:
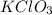
Regards!