Step-by-step explanation:
Given the amount of fluorine is ---- 1.25 mol.
What is the mass of given fluorine in grams?
Since
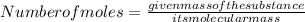
To get the mass of the substance in grams, multiply the given number of moles with the molecular mass of the substance.
Hence, among the given options, the correct answer is the last option that is
Multiply the atomic mass of fluorine by 1.25.