Step-by-step explanation:
Normality is one of the concentration terms.
It is expressed as:
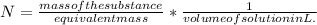
The volume of the solution is 425 mL.
Mass of sulfuric acid given is:
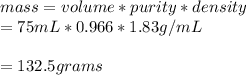
The equivalent mass of sulfuric acid is 49.0g/equivalents
Hence, the normality of the given solution is:
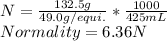
Answer is: 6.36N.