Answer:
a. increase.
Step-by-step explanation:
Hello!
In this case, according to the given information, it turns out possible for us to tell that the common ion effect decreases the solubility of the ionic solid by firstly increasing the concentration of the common ion, which is further solved for the solubility in order to evidence the aforementioned decrease.
As an example, we can consider the solubility equilibrium for silver chloride:
![Ksp=[Ag^+][Cl^-]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/ecqs5nxvjcdxws8f1t1eod.png)
Which goes to:
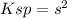
Whereas s is the solubility to be solved for. However, when a silver- or chloride-containing solution is added, say 0.1 sodium chloride, the equilibrium expression changes to:
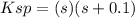
Which turns out into a smaller value for s.
Regards!