Answer:
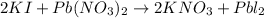
Double replacement reaction.
It is in agreement with the law of conservation of mass because we have two potassium atoms, two iodine atoms, one lead atom, two nitrogen atoms and six oxygen atoms on both sides of the chemical equation (count them).
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns possible for us to solve this problem by firstly considering that this reaction occurs between potassium iodide and lead (II) nitrate to yield potassium nitrate and lead (II) iodide which is clearly not balanced since we have one iodine atom on the reactants and two on the products, that is why the balance implies the placement of a coefficient of 2 in front of both KI and KNO3 as shown below:
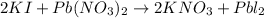
Thus, we infer this is a double replacement reaction due to the exchange of both cations, K and Pb with both anions, I and NO3. Moreover, we can tell this balanced reaction is in agreement with the law of conservation of mass because we have two potassium atoms, two iodine atoms, one lead atom, two nitrogen atoms and six oxygen atoms on both sides of the chemical equation (count them).
Regards!