Answer: If we leave liquid butane in a bowl on a table in a room where the temperature is
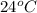 , butane will evaporate.
, butane will evaporate.
Step-by-step explanation:
A temperature at which the the liquid and gaseous phase of a substance of a substance are present in equilibrium with each other is called boiling point.
For example, the boiling point of butane is -1.5 degree Celsius.
This means that at a temperature above -1.5 degree Celsius, butane will exist is gaseous state. That is, at a temperature of 24 degree Celsius butane will evaporate.
Thus, we can conclude that if we leave liquid butane in a bowl on a table in a room where the temperature is
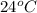 , butane will evaporate.
, butane will evaporate.