Answer:
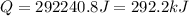
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to use the general heat equation:
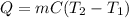
For us to plug the given mass, specific heat and temperature change to obtain the required heat:
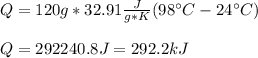
Regards!