Answer:
e. TA>T>Tc
Step-by-step explanation:
a) In this case, we cannot say for sure QA>QB>QC. This is because the magnitude of the heat flow will depend on the specific heat and the mass of each sample. Due to the equation:
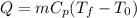
if we did an energy balance of the system, we would get that>
QA+QB+QC=0
For this equation to be true, at least one of the heats must be negative. And one of the heats must be positive.
We don't know either of them, so we cannot determine if this statement is true.
b) We can say for sure that QA<0, because when the two samples get to equilibrum, the temperatrue of A must be smaller than its original temperature. Therefore, it must have lost heat. But we cannot say for sure if QB<0 because sample B could have gained or lost heat during the process, this will depend on the equilibrium temperature, which we don't know. So we cannot say for sure this option is correct.
c) In this case we don't know for sure if the equilibrium temperature will be greater or smaller than TB. This will depend on the mass and specific heat of the samples, just line in part a.
d) is not complete
e) We know for sure that A must have lost heat, so its equilibrium temperature must be smaller than it's original temperature. We know that C must have gained heat, therefore it's equilibrium temperature must be greater than it's original temperature, so TA>T>Tc must be true.