Answer:
8.88 grams of aluminum nitrate should be weighted.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to use the definition of molarity to calculate the moles of aluminum nitrate as follows:
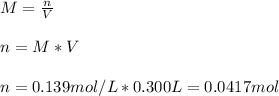
Now, since the molar mass of aluminum nitrate is 212.996 g/mol, we obtain the following mass:
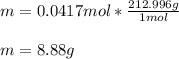
Therefore, 8.88 grams of aluminum nitrate should be weighted.
Regards!