Answer:
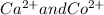
Step-by-step explanation:
Given:
A solution contains one or more of the following ions such as Ag,
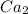 and
and
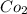
Here the Lithium bromide is added to the solution and no precipitate forms
Solution:
Since with LiBr no precipitation takes place therefore Ag+ is absent
Here on adding
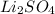 to it precipitation takes place.
to it precipitation takes place.
Precipitate is as follows,
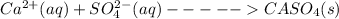
Thus,
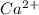 is present
is present
When
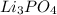 is added again precipitation takes place.
is added again precipitation takes place.
Therefore the reaction is as follows,

Therefore,
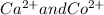 are present in the solution
are present in the solution