Answer:
By how many times would you expect Al2(SO4)3 to depress the F.P of water compared to sucrose C12H22011 ?.
Step-by-step explanation:
The freezing point of a pure solvent decreases further by adding a nonvolatile solute.
This is called depression in freezing point.
When an ionic solute is dissolved then the depression in the freezing point is proportional to the number of ions present in the solution.
In aluminum sulfate, there are five ions formed as shown below:
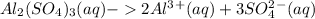
But sucrose is a covalent compound and it does not undergo dissociation.
Hence, aluminum sulfate decreases the freezing point of water by five times compared to sucrose.
Step-by-step explanation: