Answer:
8.35 × 10 ²² molecules
Step-by-step explanation:
gram molecular weight of N2O4
= 2 × 14 + 4 × 16
= 28 + 64
= 92g
1 mole N2O4 has 6 × 10²³ molecules of N2O4 and weighs 92g
therfore,
92 g has = 6 × 10²³ molecules
12.8 g has =
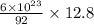
= 0.835 × 10²³
= 8.35 × 10 ²² molecules