Answer:
19.7 g.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to realize this problem can be solved by using a molecules-moles-mass relationship, starting with the given molecules, using the Avogadro's number and the molar mass of nitric acid (63.01 g/mol):
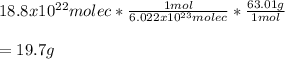
Regards!