Answer: A Bronsted-Lowry acid is defined as a substance that acts as a proton donor.
Step-by-step explanation:
A substance that is able to donate a proton or hydrogen ion to another substance is a Bronsted-Lowry acid.
For example, HCl is a Bronsted-Lowry acid as it dissociates to give a hydrogen ion.
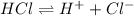
Thus, we can conclude that a Bronsted-Lowry acid is defined as a substance that acts as a proton donor.