Answer:
461.73 K
Step-by-step explanation:
Given that,
The mass of a bullet, m = 5.7 g
Speed of the bullet, v = 490 m/s
Half the kinetic energy of the bullet is transformed into internal energy and remains with the bullet while the other half is transmitted to the tree.
Using the conservation of energy,
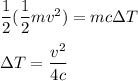
Where
x is the specific heat of lead, c = 130 J/kg K
So,
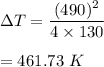
So, the increase in temperature of the bullet is 461.73 K.