Answer:
F = 1.19 x 10⁹ N
Step-by-step explanation:
The electrostatic force between the charged particles is given by Colomb's Law as follows:
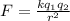
where,
F = Electrostatic force = ?
k = Colomb's constant = 9 x 10⁹ N.m²/C²
q₁ = q₂ = charge on proton = 1.6 x 10⁻¹⁹ C
r = distance between protons = 4.4 x 10⁻¹⁰ m
Therefore,
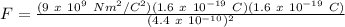
F = 1.19 x 10⁹ N