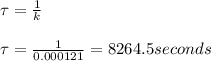Answer:
mean life = 8264.5 s
Explanation:
k = - 0.000121
The relation is given by
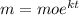
Now, the mean life is the life time for which the sample retains.
The mean life is the reciprocal of the decay constant.
The relation between the mean life and the decay constant is