Answer:
The percentage efficiency of the electrical element is approximately 82.186%
Step-by-step explanation:
The given parameters are;
The thermal energy provided by the stove element,
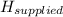 = 3.34 × 10³ J
= 3.34 × 10³ J
The amount thermal energy gained by the kettle,
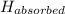 = 5.95 × 10² J
= 5.95 × 10² J
The percentage efficiency of the electrical element in heating the kettle of water, η%, is given as follows;
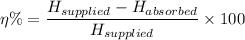
Therefore, we get;
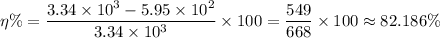
The percentage efficiency of the electrical element, η% ≈ 82.186%.