Answer:
a) Δh = 2 cm, b) Δh = 0.4 cm
Step-by-step explanation:
Let's start by using Bernoulli's equation for the Pitot tube, we define two points 1 for the small entry point and point 2 for the larger diameter entry point.
P₁ + ½ ρ v₁² + ρ g y₁ = P₂ + ½ ρ v₂² + ρ g y₂
Point 1 is called the stagnation point where the fluid velocity is reduced to zero (v₁ = 0), in general pitot tubes are used in such a way that the height of point 2 of is the same of point 1
y₁ = y₂
subtitute
P₁ = P₂ + ½ ρ v₂²
P₁ -P₂ = ½ ρ v²
where ρ is the density of fluid
now we measure the pressure on the included beforehand as a pair of communicating tubes filled with mercury, we set our reference system at the point of the mercury bottom surface
ΔP =ρ_{Hg} g h - ρ g h
ΔP = (ρ_{Hg} - ρ) g h
as the static pressure we can equalize the equations
ΔP = P₁ - P₂
(ρ_{Hg} - ρ) g h = ½ ρ v²
v =
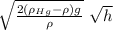
in this expression the densities are constant
v = A √h
A =
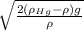
They indicate the density of mercury rhohg = 13600 kg / m³, the density of dry air at 20ºC is rho air = 1.29 kg/m³
we look for the constant
A =
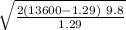
A = 454.55
we substitute
v = 454.55 √h
to calculate the uncertainty or error of the velocity
h =
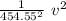
Δh =
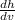 Δv
Δv
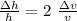
Suppose we have a height reading of h = 20 cm = 0.20 m
a) uncertainty 2.5 m / s ( 0.05)
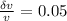
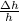 = 2 0.05
= 2 0.05
Δh = 0.1 h
Δh = 0.1 20 cm
Δh = 2 cm
b) uncertainty 0.5 m / s ( Δv/v= 0.01)
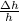 = 2 0.01
= 2 0.01
Δh = 0.02 h
Δh = 0.02 20
Δh = 0.1 20 cm
Δh = 0.4 cm = 4 mm