Answer:
pKa = 4.89.
Step-by-step explanation:
We can solve this problem by using the Henderson-Hasselbach equation, which states:
pH = pKa + log
![([A^-])/([HA])](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/6wznjdxsb2uzxomlsas3t9.png)
In this case [A⁻] is the concentration of sodium benzoate and [HA] is the concentration of benzoic acid.
We input the given data:
4.63 = pKa + log
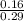
And solve for pKa:
pKa = 4.89