Step-by-step explanation:
This is a decomposition reaction. Firstly, you will want to write the chemical equation out and balance it.
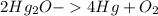 (The -> is supposed to be an arrow, sorry!)
(The -> is supposed to be an arrow, sorry!)
We see that there's only 1mol of Oxygen made in the products, we can do some simple math to solve for the amount of grams of Oxygen produced according to the amount of the reactant (Hg2O).
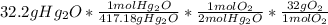
I want to break this down, just in case:
The 417.18gHg2O is the molecular mass of the molecule (so I doubled Hg and added 16 to it to get this number).
As we can see in the chemical equation, 1mol Hg2O produces 2mol O because Oxygen is a diatomic molecule (so there will always be two of it when it's by itself).
And finally, in 1mol O2 there are 32g of O2.
** When you do math like this, always make sure that all of your units cancel out except for the units you're looking for. For example, here we're looking for the grams of Oxygen, so after everything else cancels out, we should only have grams O2.
So, 1.23gO2 should be your answer.