Answer:
How many distinct monochlorinated products can result when methylcyclohexane is subjected to free radical chlorination under UV light.
Step-by-step explanation:
Consider 1-methylcyclohexane:
Its structure is shown below:
It has primary
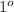 , secondary
, secondary
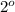 and tertiary
and tertiary
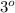 carbons as shown in the image.
carbons as shown in the image.
So, the following mono chlorinated product will be formed.