Answer:
The maximum mass of water that could be produced by the chemical reaction=0.441g
Step-by-step explanation:
We are given that
Given mass of HBr=5.7 g
Given mass of sodium hydroxide=0.980 g
Molar mass of HBr=80.9 g/ Mole
Molar mass of NaOH=40 g/mole
Molar mass of H2O=18 g/mole
Reaction

Number of moles=
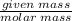
Using the formula
Number of moles of HBr=
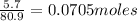
Number of moles of NaOH=
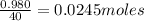
Hydrogen bromide is in a great excess and the amount of water produced.
Therefore,
Number of moles of water, n(H2O)=Number of moles of NaOH=0.0245moles
Now,
Mass of water=
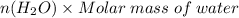
Mass of water=
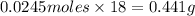
Hence, the maximum mass of water that could be produced by the chemical reaction=0.441g