Answer:
6.0 mol LiNO₃
Step-by-step explanation:
- 3Ca(NO₃)₂ + 2Li₃PO₄ → 6LiNO₃ + Ca₃(PO₄)₂
First we need to determine which reactant is the limiting reactant:
We calculate with how many Ca(NO₃)₂ moles would 3.0 moles of Li₃PO₄ react, using the stoichiometric coefficients of the reaction:
- 3.0 mol Li₃PO₄ *
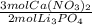 = 4.5 mol Ca(NO₃)₂
= 4.5 mol Ca(NO₃)₂
There are not as many Ca(NO₃)₂ moles, so Ca(NO₃)₂ is the limiting reactant.
Using the number of moles of the limiting reactant, we calculate how many moles of LiNO₃ would be formed:
- 3.0 mol Ca(NO₃)₂ *
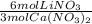 = 6.0 mol LiNO₃
= 6.0 mol LiNO₃