The question is incomplete, the complete question is:
What volume (mL) of the sweetened tea described in Example 3.14 contains the same amount of sugar (mol) as 10 mL of the soft drink in this example. The example is attached below.
Answer: 75 mL of sweetened tea will contain the same amount of sugar as in 10 mL of soft drink
Step-by-step explanation:
We first calculate the number of moles of soft drink in a volume of 10 mL
The formula used to calculate molarity:
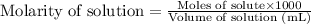 .....(1)
.....(1)
Taking the concentration of soft drink from the example be = 0.375 M
Volume of solution = 10 mL
Putting values in equation 1, we get:
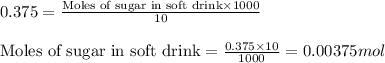
Calculating volume of sweetened tea:
Moles of sugar = 0.00375 mol
Molarity of sweetened tea = 0.05 M
Putting values in equation 1, we get:
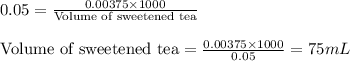
Hence, 75 mL of sweetened tea will contain the same amount of sugar as in 10 mL of soft drink