Answer:
ΔT = 25°C
Step-by-step explanation:
Given that.
The mass of a bullet, m₁ = 30 g = 0.03 kg
The speed of the bullet, v = 900 m/s
Mass of soft iron, m₂ = 1 k
The specific heat of iron, c=490J/kg°C
We need to find the increase in temperature of iron. using the conservation of energy,
Kinetic energy = heat absorbed
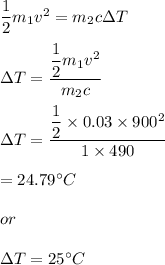
So, the correct option is (A).