Answer:
Step-by-step explanation:
The equation for Coulomb's Law is
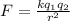 where k is Coulomb's constant, q1 is one of the charges, q2 is another one of the charges (1 of these has to be the one in question, so we will let that be q2) and r is the distance between them squared.
where k is Coulomb's constant, q1 is one of the charges, q2 is another one of the charges (1 of these has to be the one in question, so we will let that be q2) and r is the distance between them squared.
First thing we are going to do is convert those microCoulombs to Coulombs (C).
q1:
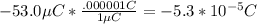 and
and
q2:
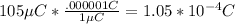 and
and
q3: our main particle that we will put in for q2 in the formula converts as follows:
q3:
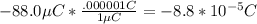
First we will find the charge between q1 and the main particle:
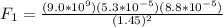 Notice that we did not use the negative charges here. We take the negative charge into account depending upon whether or not the charges are repelled or attracted. Both of these charges are negative, so they will repel and the answer will be made negative. Finding the first force:
Notice that we did not use the negative charges here. We take the negative charge into account depending upon whether or not the charges are repelled or attracted. Both of these charges are negative, so they will repel and the answer will be made negative. Finding the first force:
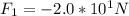 (negative because they repel so q1 will move away from the charge in question, which is also negative)
(negative because they repel so q1 will move away from the charge in question, which is also negative)
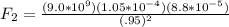 and the charge between these is
and the charge between these is
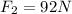 and that is to the right, so positive. These charges are opposite, so they attract. The net force is the sum of the forces, so:
and that is to the right, so positive. These charges are opposite, so they attract. The net force is the sum of the forces, so:
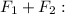 -2.0 × 10¹ + 92 = 72N (to the right)
-2.0 × 10¹ + 92 = 72N (to the right)