Answer: The value of
![[H_(3)O^(+)]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/6kp9iluw1negs122hdg3fc.png) is
is
 M and
M and
![[OH^(-)]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/r68havuojue4160c3u38rl.png) is
is
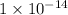 .
.
Step-by-step explanation:
pH is the negative logarithm of concentration of hydrogen ion.
It is given that pH is 11.22. So, the value of concentration of hydrogen ions is calculated as follows.
![pH = - log [H^(+)]\\11.22 = - log [H^(+)]\\conc. H^(+) = 6.025 * 10^(-12)](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/2dw3murmri4xm1p2lqqhea.png) M
M
Let the value
 is considered as equal to 0. Hence, the relation between pH and pOH value is as follows.
is considered as equal to 0. Hence, the relation between pH and pOH value is as follows.
pH + pOH = 14
0 + pOH = 14
pOH = 14
Now, pOH is the negative logarithm of concentration of hydroxide ions.
Hence,
![[OH^(-)]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/r68havuojue4160c3u38rl.png) is calculated as follows.
is calculated as follows.
![pOH = - log [OH^(-)]\\14 = - log [OH^(-)]\\conc. OH^(-) = 1 * 10^(-14) M](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/9ij0svox355749tadx6cea.png)
Thus, we can conclude that the value of
![[H_(3)O^(+)]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/6kp9iluw1negs122hdg3fc.png) is
is
 M and
M and
![[OH^(-)]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/r68havuojue4160c3u38rl.png) is
is
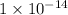 .
.