Answer: The number of moles of NaOH is 0.0036 moles
Step-by-step explanation:
Molarity is defined as the amount of solute expressed in the number of moles present per liter of solution. The units of molarity are mol/L. The formula used to calculate molarity:
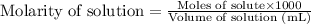 .....(1)
.....(1)
Given values:
Molarity of NaOH =
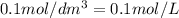 (Conversion factor:
(Conversion factor:
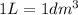
Volume of the solution =
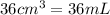 (Conversion factor:
(Conversion factor:
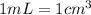
Putting values in equation 1, we get:
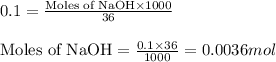
Hence, the number of moles of NaOH is 0.0036 moles