Answer:
For a: The balanced equation is
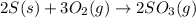
For c: The balanced equation is
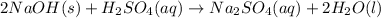
Step-by-step explanation:
A balanced chemical equation is one where all the individual atoms are equal on both sides of the reaction. It follows the law of conservation of mass.
The given unbalanced equation follows:
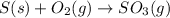
To balance the equation, we must balance the atoms by adding 2 infront of both
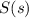 and
and
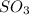 and 3 in front of
and 3 in front of
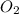
For the balanced chemical equation:
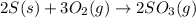
The given balanced equation follows:
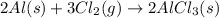
The given equation is already balanced.
The given unbalanced equation follows:
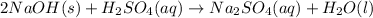
To balance the equation, we must balance the atoms by adding 2 infront of
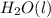
For the balanced chemical equation:
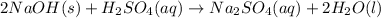
The given balanced equation follows:
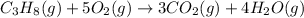
The given equation is already balanced.