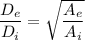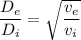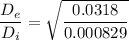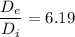Solution :
For R-134a, we are given :
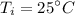
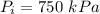
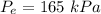
Now we have one inlet and one exit flow, no work and no heat transfer. The energy equation is :
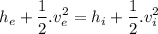
We also know that the gas is throttled and there is no change in the kinetic energy.
So,
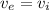
Now from the energy equation above, we can see that the inlet and the exit enthalpies are also the same. Therefore,
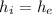
From the saturated R-134a table, corresponding to
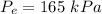 , we can find the exit saturation temperature.
, we can find the exit saturation temperature.

From the saturated R-134a table, corresponding to
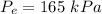 , we can find the specific enthalpies :
, we can find the specific enthalpies :
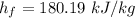
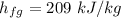
Calculating the exit flow quality factor,
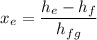
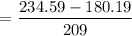
= 0.26
From the saturated R-134a table, corresponding to
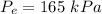 , we can find the specific volumes :
, we can find the specific volumes :
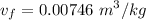
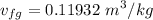
Calculating the exit specific volume :
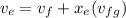
= 0.000746 + 0.26 (0.11932)
= 0.0318
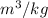
The mass flow is equal to :
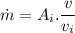
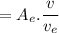
So,
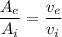
Therefore, the ratio of the exit pipe and the inlet pipe diameter is equal to