Answer:
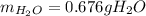
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the maximum mass of water by firstly setting up the undergoing chemical reaction as follows:
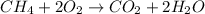
Thus, we are able to firstly calculate the moles of water produced by both methane and oxygen in order to identify the limiting reactant, which is related to maximum of water:
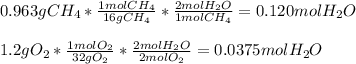
Thus, we infer the limiting reactant is O2 and therefore we can obtain up to 0.0375 moles of water, which are related to the following mass:
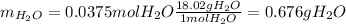
Regards!