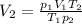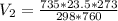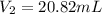Complete Question
4. A piece of metal weighing 0.0713 g was placed in a eudiometer containing dilute aqueous HCl. After the metal fully dissolved, 23.5 mL of hydrogen gas was collected by displace-ment of water and a 400 mm column of water was observed. The water temperature was 258C and the barometric pressure was 758.8 mm Hg (torr). Refer to the Introduction and data sheet to solve the following problems.
a) What is the vapor pressure of the water vapor in the column? (Consult Appendix E.)
b) What is the pressure of the water column expressed in mm Hg (torr)? The density of mercury is 13.6 g/mL.
c) Calculate the pressure of the hydrogen gas above the water in the column.
d) Calculate the volume occupied by the hydrogen gas at STP.
Answer:
a)
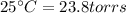
b)
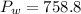
c)
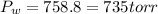
d)
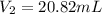
Step-by-step explanation:
From the question we are told that:
Metal weight
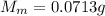
Volume Hydrogen
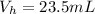
Displace-ment Column of water 400 mm column of water
Temperature
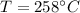
Barometric Pressure
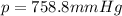
Vapour Pressure of water at
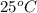
Generally from (Consult Appendix E.)
a)
Va-pour Pressure of water at
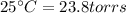
b)
Pressure of Water column
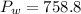
c) Pressure of Water column (Consult Appendix E.)
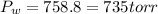
d)
Generally the equation for ideal gas is mathematically given by
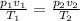
Therefore