Answer: The given pairs of electrons most likely reside in
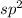 type of orbital.
type of orbital.
Step-by-step explanation:
As it is given that two lone pair of electrons are present on the oxygen atom of ketone (such as cyclohexanone).
Also, there will be one bond pair between carbon and oxygen atom.
Hence, total electrons present in the domain are as follows.
2 lone pairs + 1 bon pair of electron = 3 electron domains
This means that there will be
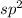 type of orbital present.
type of orbital present.
Thus, we can conclude that given pairs of electrons most likely reside in
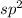 type of orbital.
type of orbital.