Answer:
e) ⁵²Cr
Step-by-step explanation:
The general form of alpha decay is as follows:
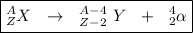 .
.
From this, we can see that during alpha decay, the mass number decreases by 4 and the atomic number decreases by 2.
Therefore, we need to find a nucleus that has 4 more nucleons (i.e., a mass number that is 4 more) than that of Ti-48, which is 48 + 4 = 52.
The only option with a nuclear number of 52 is ⁵²Cr, and therefore, Ti-48 is produced by the alpha decay of ⁵²Cr.