Answer: it will have a pressure of approximately 371.73 κPa.
We know that According to Boyle's Law
P1 ×V1 = P2 ×V2
therefore in order to find P2
P2 =
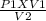 --------------------(I)
--------------------(I)
where
P1 = initial pressure
V1 = initial volume
P2 = final pressure
V2 = final volume
here
P1(initial pressure) = 146 kPa
V1(initial volume) = 2.55 L
V2(final volume) = 2 L
Now putting the given values in equation (I) :
P2 = (146 kPa × 2.55 L) / 2 L
P2 = 371.73 kPa
Therefore, 2 L of gas, when it takes 2.55 L at 146 kPa, will have a pressure of 371.73 kPa.