Given :
Number of He atoms,
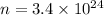 atoms.
atoms.
To Find :
How many grams are their in given number of He atoms.
Solution :
We know, molecular mass of He is 4 g. It means that their are
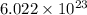 atoms in 4 g of He.
atoms in 4 g of He.
Let, number of gram He in
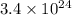 atoms is x , so :
atoms is x , so :
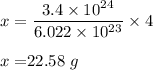
Therefore, grams of He atoms is 22.58 g .