The density of the given ball is 0.808. Density is an intrinsic property of an object.
How to calculate density?
The density of a ball can be calculated by diving its mass by its volume. It can written as,
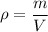
Where,
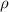 - density
- density
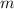 - mass = 404.2 g
- mass = 404.2 g
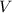 - volume = 500.0 cm³
- volume = 500.0 cm³
Put the values in the formula,
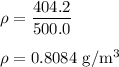
Therefore, the density of the given ball is 0.808.
Learn more about density: