Solution :
Volume of balloon
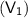 = 6.7 L
= 6.7 L
Temperature
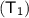 = 20 celsius (converting into kelvin) = 20 + 273 = 293 K
= 20 celsius (converting into kelvin) = 20 + 273 = 293 K
Now,
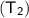 = 350 celsius
= 350 celsius
= 350 + 273 = 623 K
Since pressure is constant.
According to Charles law, At constant pressure the volume of the gas is directly proportional to temperature.
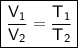
We have to find

On putting the values in above formula,,
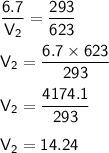
Therefore, Volume of the balloon will be 14.24 L at 350 celsius.