Answer:
0.059 moles are present in 2.6 g of CO₂
Step-by-step explanation:
Molar mass is the mass of one mole of a substance, which can be an element or a compound. That is, it is the amount of mass that a substance contains in one mole.
In this case, the mass of each element being:
then the molar mass of the compound is:
CO₂= 12 g/mole + 2*16 g/mole= 44 g/mole
Then you can apply the following rule of three: if by definition of molar mass 44 grams of CO₂ are present in 1 mole, 2.6 grams will be present in how many moles?
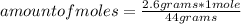
amount of moles= 0.059 moles
0.059 moles are present in 2.6 g of CO₂