Answer:
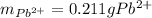
Step-by-step explanation:
Hello!
In this case, according to the stoichiometry of the reaction, it is possible to evidence the 1:1 mole ratio between lead (II) ions and lead (II) sulfate precipitate; that is why we can compute the mass of lead (II) in the polluted water as shown below:
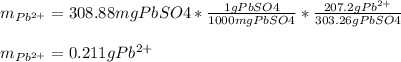
Best regards!