Answer:
3 mol AlCl₃.
Step-by-step explanation:
Hello!
In this case, according to the specified reactants and products, it is possible to set up the following balanced chemical reaction:
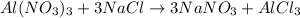
Whereas we evidence the 1:3 mole ratio between aluminum nitrate and sodium chloride; thus, since different moles were reacting, we need to identify the limiting reactant by computing the moles of AlCl₃ produced by each reactant as follows:
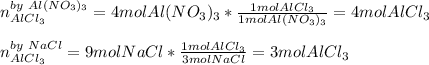
Thus, we infer that NaCl is the limiting reactant as it produces the fewest moles of AlCl₃; consequently the produced amount of this product is 3 mol.
Best regards!