Answer:
69.647g Ag
Step-by-step explanation:
First, determine the molecular weight of
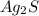 :
:
Atomic Mass Ag = 107.87
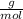
Atomic Mass S = 32.07
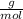
So,
Molecular Weight
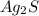 = 2(107.87
= 2(107.87
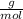 )+(32.07
)+(32.07
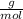 )
)
Molecular Weight
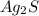 =247.81
=247.81
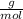
Then determine the mass of silver contained in the sample:
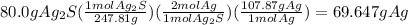
This answer makes sense because the mass of silver in the compound must be less than the total mass of the compound.