Answer:
C. 43.9 g
Step-by-step explanation:
The formula for calculating the heating of the water:
Q = m * C * ΔT
Where Q is the amount of heat required, m is the mass of the object, C is the specific heat capacity of the material, and ΔT is the change in temperature. Plugging in the values from the problem, we get the following:
Q = 4000 g * 4.184 J/g°C * (100°C - 20°C) = 1,338,880 J = 1338.88 kJ
The mass of anthracite coal required:
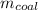 = 1338.88 kJ / 30,500 kJ/kg = 0.0439 kg = 43.9 g
= 1338.88 kJ / 30,500 kJ/kg = 0.0439 kg = 43.9 g