The wavelength of the electrons is obtained as 0.087 A°.
The de Broglie wavelength is a concept in quantum mechanics introduced by Louis de Broglie. It suggests that particles, such as electrons, exhibit both particle and wave-like properties.
The threshold energy is 3ev or 4.8 *
 J
J
The energy of the light;
E = hc/λ
E = 6.6 *
 * 3 *
* 3 *
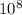 /248 *
/248 *

E = 7.98 *
 J
J
KE = 7.98 *
 J - 4.8 *
J - 4.8 *
 J
J
= 3.18 *
 J
J
The velocity of the electrons= √2KE/m
= √2 * 3.18 *
 /9.11 *
/9.11 *

= 8.35 *
 m/s
m/s
But momentum = mv
λ= h/mv
λ= 6.6 *
 /9.11 *
/9.11 *
 * 8.35 *
* 8.35 *

= 8.7 *
 m or 0.087 A°
m or 0.087 A°